
Chemistry, 23.01.2020 03:31 coollid876
Fe(s) + 2hcl(aq) --> fecl2(aq) + h2(g)
when a student adds 30.0 ml of 1.00 m hcl to 0.56 g of powdered fe, a reaction occurs according to the equation above. when the reaction is complete at 273 k and 1.0 atm, which of the following is true?
a) hcl is in excess, and 0.100 mol of hcl remains unreacted.
d) 0.22 l of h2 has been produced.
the correct answer is d. i can't figure out why a is wrong.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1
You know the right answer?
Fe(s) + 2hcl(aq) --> fecl2(aq) + h2(g)
when a student adds 30.0 ml of 1.00 m hcl to...
when a student adds 30.0 ml of 1.00 m hcl to...
Questions



Law, 07.11.2021 01:20

Chemistry, 07.11.2021 01:20

Mathematics, 07.11.2021 01:40

Mathematics, 07.11.2021 01:40



History, 07.11.2021 01:50




Mathematics, 07.11.2021 02:20





Mathematics, 07.11.2021 02:50


Arts, 07.11.2021 03:00

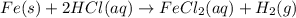
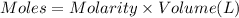
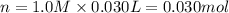
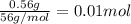
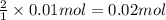 of HCl
of HCl

