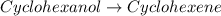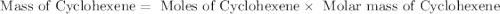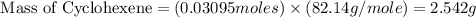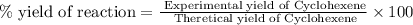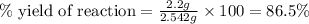Consider the reaction of cyclohexanol and an acid catalyst to obtain cyclohexene.
cyclohexanol (100.16 g/mol) --> cyclohexene (82.14 g/mol)
a reaction was performed in which 3.1 g of cyclohexanol was reacted with an acid catalyst to obtain 2.2 g of cyclohexene. calculate the percent yield for this reaction (in %).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

You know the right answer?
Consider the reaction of cyclohexanol and an acid catalyst to obtain cyclohexene.
cycloh...
cycloh...
Questions

Mathematics, 28.05.2020 22:59

Biology, 28.05.2020 22:59

History, 28.05.2020 22:59

Mathematics, 28.05.2020 22:59



English, 28.05.2020 22:59


Biology, 28.05.2020 22:59


Mathematics, 28.05.2020 22:59


History, 28.05.2020 22:59




Mathematics, 28.05.2020 22:59



Mathematics, 28.05.2020 22:59