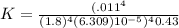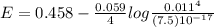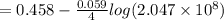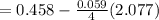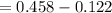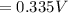Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Express the following number in scientific notation. 0.026890 =
Answers: 1


Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 23.06.2019 05:30
The image compares the arrangement of electrons in two different neutral atoms. a figure labeled atom q has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has six black spheres. to the left of this figure is another figure labeled atom p. atom p has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has seven black spheres. which of the following best explains the position of the two atoms in the periodic table? atom p has an estimated zeff of 7 and is therefore to the left of atom q, which has a zeff of 6. atom p has an estimated zeff of 7 and is therefore to the right of atom q, which has a zeff of 6. atom p has an estimated zeff of 5 and is therefore below atom q, which has a zeff of 4. atom p has an estimated zeff of 5 and is therefore above atom q, which has a zeff of 4.
Answers: 3
You know the right answer?
What is the emf of this cell when [fe2+]= 1.8 m , [fe3+]= 1.1×10−2 m, po2= 0.43 atm and the ph of th...
Questions

Engineering, 07.03.2021 04:40




Mathematics, 07.03.2021 04:40

Mathematics, 07.03.2021 04:40



Mathematics, 07.03.2021 04:40





Computers and Technology, 07.03.2021 04:40



Computers and Technology, 07.03.2021 04:40

Mathematics, 07.03.2021 04:50

World Languages, 07.03.2021 04:50

Health, 07.03.2021 04:50

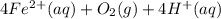 →
→ 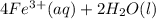
![E= E^{0} -\frac{nF}{RT} ln \frac{[Products]}{[Reactants]} \\\\\\E= E^{0} -2.303\frac{nF}{RT} log \frac{[Products]}{[Reactants]}\\\\\\E= E^{0} -\frac{0.059}{n} log \frac{[Products]}{[Reactants]}](/tpl/images/0455/5861/590e3.png)
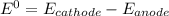
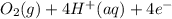 →
→ 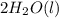 , the value of reduction potential =1.229V
, the value of reduction potential =1.229V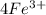 →
→ 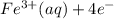 , the value of reduction potential =0.771 V
, the value of reduction potential =0.771 V
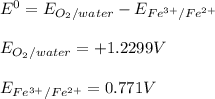
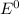 = 1.229 - 0.771 = 0.458 V
= 1.229 - 0.771 = 0.458 V
![[Fe^{3+}]=0.011 M\\ [Fe^{2+}]=1.8 M](/tpl/images/0455/5861/b03a8.png)
![[H^+]](/tpl/images/0455/5861/07acb.png)
![[H^+]=6.309*10^{-5} M](/tpl/images/0455/5861/21d19.png)
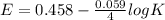
![K=\frac{[Fe^{3+}]^4}{[Fe^{2+}]^4 [H^+]^4 pO_2}](/tpl/images/0455/5861/b8f59.png)
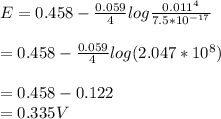
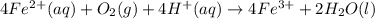
![E = E^{0}-\frac{RT}{nF}ln\frac{[Product]}{[Reactant]}](/tpl/images/0455/5861/e9c22.png)
![E = E^{0}-2.303\frac{RT}{nF}log\frac{[Product]}{[Reactant]}](/tpl/images/0455/5861/56449.png)
![E = E^{0}-\frac{0.059}{n}log\frac{[Product]}{[Reactant]}](/tpl/images/0455/5861/238b6.png)
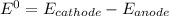
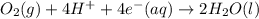 .......1.229 V
.......1.229 V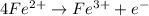 .........0.771 V
.........0.771 V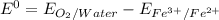
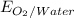 = +1.229 V (from standard reduction potential table)
= +1.229 V (from standard reduction potential table)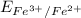 = 0.771 V
= 0.771 V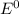 = 1.229 - 0.771 = 0.458 V
= 1.229 - 0.771 = 0.458 V![[Fe^{3+}]= 1.1\times 10^{-2}M](/tpl/images/0455/5861/68a68.png) = 0.011 M
= 0.011 M![[Fe^{2+}]= 1.8M](/tpl/images/0455/5861/574f9.png)
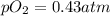
![[H^{+}]= 6.309\times 10^{-5}M](/tpl/images/0455/5861/f95e6.png)
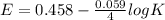
![K =\frac{[Fe^{3+}]^{4}}{[Fe^{2+}]^{4}[H+]^{4}pO_{2}}](/tpl/images/0455/5861/4219c.png)