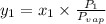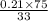Chemistry, 14.01.2020 20:31 danielamejia13
At 20 °c, the vapor pressure of benzene(c6h8) is 75 torr, and that of toluene(c7h8) is 22 torr. assuming that benzene and toluene from and idea solution.
a) what is the composition in mole fractions of a solution that has a vapor pressure of 33 torr at 20 °c
b) what is the mole fraction of benzene in the vapor above the solution described in part a?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2


Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1

Chemistry, 23.06.2019 15:30
Sodium chloride can be made as follows: 2na + cl2 ? 2nacl i calculate the maximum amount of nacl possible if 2.3 g of sodium was reacted with excess chlorine. show all your workings.
Answers: 3
You know the right answer?
At 20 °c, the vapor pressure of benzene(c6h8) is 75 torr, and that of toluene(c7h8) is 22 torr. assu...
Questions








Mathematics, 03.06.2020 01:57

History, 03.06.2020 01:57

Mathematics, 03.06.2020 01:57




Biology, 03.06.2020 01:57




Mathematics, 03.06.2020 01:57

Mathematics, 03.06.2020 01:57

History, 03.06.2020 01:57

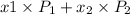
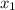 = mole fraction of component one
= mole fraction of component one
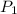 = vapor pressure of component one when pure
= vapor pressure of component one when pure
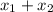 = 1
= 1
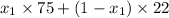
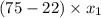
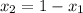
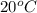 is 0.79.
is 0.79.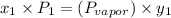
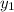 = composition in gas phase
= composition in gas phase