
Chemistry, 09.01.2020 07:31 brooke7768
Agas mixture with 4 mol of ar, x moles of ne, and y moles
ofxe is prepared at a pressure of 1 bar and a temperature of
298k. the total number of moles in the mixture is three times thatof
ar. write an expression for the deltagmixing in termsof
x. at what value of x does the magnitude ofdeltagmixing
have its maximum value? calculatedeltagmixing for this
value of x.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:50
2points why do scientists need governmental funding? o a. government politicians ask all the important scientific questions. o b. scientists have to pay taxes to the government on the money they make. o c. the cost of doing scientific research can be very high. o d. the government is controlled by scientists. submit
Answers: 3

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1
You know the right answer?
Agas mixture with 4 mol of ar, x moles of ne, and y moles
ofxe is prepared at a pressure of 1...
ofxe is prepared at a pressure of 1...
Questions






Social Studies, 14.03.2020 02:43


History, 14.03.2020 02:44



Computers and Technology, 14.03.2020 02:44






History, 14.03.2020 02:44

Mathematics, 14.03.2020 02:44

Mathematics, 14.03.2020 02:44

![\Delta G_{mixing}=\frac{R*T}{12}*[4*ln (1/3) +x*ln (x/12) +(8-x)*ln ((8-x)/12)]](/tpl/images/0448/2362/a32a1.png)
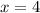
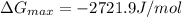
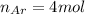

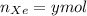
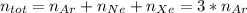
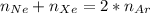
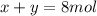
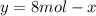
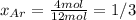
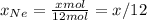
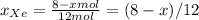
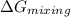
![\Delta G_{mixing}=R*T*\sum_{i]*x_i*ln (x_i)](/tpl/images/0448/2362/afcae.png)
![\Delta G_{mixing}=R*T*[1/3*ln (1/3) +x/12*ln (x/12) +(8-x)/12*ln ((8-x)/12)]](/tpl/images/0448/2362/cbb53.png)
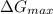
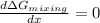
![\frac{d \Delta G_{mixing}}{dx}=\frac{R*T}{12}*[ln (x/12)+12-ln ((8-x)/12)-12]](/tpl/images/0448/2362/0152c.png)
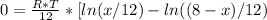
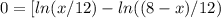
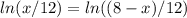
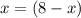
![\Delta G_{max}=\frac{8.314*298}{12}*[4*ln (1/3) +4*ln (4/12) +(8-4)*ln ((8-4)/12)]](/tpl/images/0448/2362/42253.png)
![\Delta G_{max}=\frac{8.314*298}{12}*[4*ln (1/3) +4*ln (1/3) +(4)*ln (1/3)]](/tpl/images/0448/2362/c470a.png)
![\Delta G_{max}=\frac{8.314*298}{12}*[12*ln (1/3)]](/tpl/images/0448/2362/4c885.png)


