Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1
You know the right answer?
A Assuming the reaction is first order in sucrose, determine the mass of sucrose that is hydrolyzed...
Questions

Mathematics, 30.04.2021 07:50

History, 30.04.2021 07:50

Biology, 30.04.2021 07:50

Chemistry, 30.04.2021 07:50

Computers and Technology, 30.04.2021 07:50




Mathematics, 30.04.2021 07:50




Mathematics, 30.04.2021 07:50

Mathematics, 30.04.2021 07:50


Geography, 30.04.2021 07:50

Chemistry, 30.04.2021 07:50



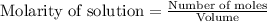
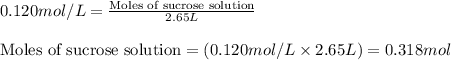
![k=\frac{2.303}{t}\log\frac{[A_o]}{[A]}](/tpl/images/0547/4142/f1041.png)
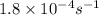
![[A_o]](/tpl/images/0547/4142/dc622.png) = initial amount of the sample = 0.318 moles
= initial amount of the sample = 0.318 moles![1.8\times 10^{-4}=\frac{2.303}{12000}\log\frac{0.318}{[A]}](/tpl/images/0547/4142/9391b.png)
![[A]=0.0367mol](/tpl/images/0547/4142/40b69.png)