

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3

Chemistry, 23.06.2019 01:30
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1
You know the right answer?
Calculate the lattice energy of kcl(s) given the following data using the born-haber cycle: δhsubli...
Questions









Mathematics, 31.07.2020 17:01



Mathematics, 31.07.2020 17:01



Mathematics, 31.07.2020 17:01

Mathematics, 31.07.2020 17:01


Chemistry, 31.07.2020 17:01


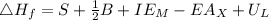
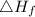 is enthalpy of formation
is enthalpy of formation 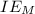 is ionisation enthalpy of metal
is ionisation enthalpy of metal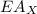 is electron affinity of non metal atom
is electron affinity of non metal atom 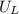 is lattice energy
is lattice energy 

