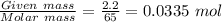Chemistry, 31.12.2019 05:31 carlosleblanc26
Zn + 2hcl → zncl2 + h2
at conditions of standard temperature and pressure, determine how many liters of hydrogen gas are produced by placing a zinc nail with a mass of 2.2g into an excess of hydrochloric acid.
a) 0.0015 l b) 0.75 l c) 1.33 l d) 6.42 l

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2
You know the right answer?
Zn + 2hcl → zncl2 + h2
at conditions of standard temperature and pressure, determine how many...
at conditions of standard temperature and pressure, determine how many...
Questions

Mathematics, 29.12.2019 22:31

English, 29.12.2019 22:31

Mathematics, 29.12.2019 22:31

Mathematics, 29.12.2019 22:31



Mathematics, 29.12.2019 22:31


Biology, 29.12.2019 22:31



Chemistry, 29.12.2019 22:31

Health, 29.12.2019 22:31


Mathematics, 29.12.2019 22:31



Mathematics, 29.12.2019 22:31


English, 29.12.2019 22:31