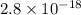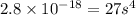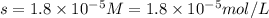Chemistry, 31.12.2019 04:31 babygirl10302015
The solubility product ag3po4 is: ksp = 2.8 x 10^-18. what is the solubility of ag3po4 in water, in moles per liter?

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 07:00
The following transition occurs at a molecular level for a substance. what transition corresponds to this change in microscopic structure? the carbon dioxide molecules on the left are in a regular, tightly packed pattern. after heating, it becomes much lower density. a. melting b. boiling c. sublimation d. freezing
Answers: 1

Chemistry, 23.06.2019 09:00
What factor besides temperature affects the boiling point of water? a. mass b. number of moles c. volume d. pressure
Answers: 3

Chemistry, 23.06.2019 09:20
La reaccion entre monoxido de nitrogeno (no) y oxigeno para formardioxido de nitrogeno (no2) es un paso determinante para la formacion del smog, la reaccion es la siguiente: 2no + o2 = 2no2 cual sera el numero de moles de no2 que se formaran por la reaccion completa de 8 moles de oxigeno con suficiente monoxido?
Answers: 1

Chemistry, 23.06.2019 10:00
Two moles of potassium chloride and three moles of oxygen are produced from the decomposition of two moles of potassium chlorate, kcos3(s). write the balanced equation. how many moles of oxygen are produced from 12 moles of potassium chlorate
Answers: 1
You know the right answer?
The solubility product ag3po4 is: ksp = 2.8 x 10^-18. what is the solubility of ag3po4 in water, in...
Questions

Mathematics, 23.07.2021 06:20


Mathematics, 23.07.2021 06:20

Chemistry, 23.07.2021 06:20



Biology, 23.07.2021 06:20



Social Studies, 23.07.2021 06:20

Chemistry, 23.07.2021 06:20


Mathematics, 23.07.2021 06:20

Mathematics, 23.07.2021 06:20

Mathematics, 23.07.2021 06:20

Mathematics, 23.07.2021 06:20


Mathematics, 23.07.2021 06:20


Social Studies, 23.07.2021 06:20

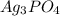 in water is,
in water is, 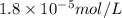
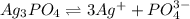
![K_{sp}=[Ag^{+}]^3[PO_4^{3-}]](/tpl/images/0437/9779/7aeef.png)
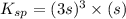
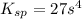
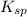 =
=