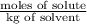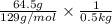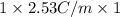Chemistry, 27.12.2019 03:31 douglasally
Assuming ideal behavior, calculate the boiling point in c of solution that contains 64.5g or the non-volatile non-electrolyte quinoline( molar mass= 129g/mole) in 500 grams of benzene. for benzene the normal boling point is 80.10 c and kb = 2.53c/m

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Concerning the 10.0 ml of 0.50 m nacl to 100 ml of solution: when a solution is diluted, does it change the number of moles dissolved?
Answers: 3


Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3
You know the right answer?
Assuming ideal behavior, calculate the boiling point in c of solution that contains 64.5g or the non...
Questions


Mathematics, 08.04.2021 06:10





Mathematics, 08.04.2021 06:10







Medicine, 08.04.2021 06:10

English, 08.04.2021 06:10


Mathematics, 08.04.2021 06:10



Mathematics, 08.04.2021 06:10

 ,
, 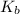 = 2.53 C/m
= 2.53 C/m