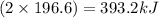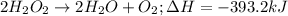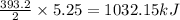Chemistry, 26.12.2019 21:31 swaggg7108
The decomposition of hydrogen peroxide to form water and oxygen gas releases 196.6 kj per mole of hydrogen peroxide. this reaction occurs when hydrogen peroxide is placed on a skin cut to sterilize it. how much heat is released when 5.25 moles of h2o2 decompose?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 23.06.2019 02:30
When the ionic compound nabr dissolves in water, br– ions are pulled into solution by the attraction between what two particles? a. the na+ and br– ions b. the na+ ion and the negative end of a water molecule c. the br– ion and the positive end of a water molecule d. the br– ion and the negative end of a water molecule
Answers: 1
You know the right answer?
The decomposition of hydrogen peroxide to form water and oxygen gas releases 196.6 kj per mole of hy...
Questions

Mathematics, 07.10.2019 02:50



English, 07.10.2019 02:50

Mathematics, 07.10.2019 02:50

History, 07.10.2019 02:50




History, 07.10.2019 02:50

Mathematics, 07.10.2019 02:50

History, 07.10.2019 02:50

Chemistry, 07.10.2019 02:50