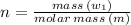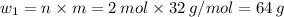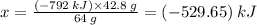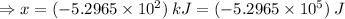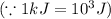Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2
You know the right answer?
Sulfur undergoes combustion to yield sulfur trioxide by the following reaction equation:
Questions

Mathematics, 22.02.2021 01:20


English, 22.02.2021 01:20




Mathematics, 22.02.2021 01:20



English, 22.02.2021 01:20

Computers and Technology, 22.02.2021 01:20

Mathematics, 22.02.2021 01:20




Chemistry, 22.02.2021 01:20

Mathematics, 22.02.2021 01:20

Arts, 22.02.2021 01:20

Chemistry, 22.02.2021 01:20