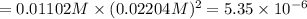Chemistry, 23.12.2019 17:31 puchie1225
Excess ca(oh)₂ is shaken with water to produce a saturated solution. the solution is filtered, and a 50.00 ml sample titrated with hcl requires 11.21 ml of 0.0983 m hcl to reach the end point.
calculate  for ca(oh)₂. compare your result with that in appendix d. do you think the solution was kept at 25°c?
for ca(oh)₂. compare your result with that in appendix d. do you think the solution was kept at 25°c?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 23.06.2019 18:00
What would make it possible for magnesium atom to have a noble gas configuration
Answers: 3

Chemistry, 23.06.2019 19:00
How can evidence from an experiment be explained in her relationship to the hypothesis? a.as a prediction b.as a question c.as an in inference d.as a conclusion
Answers: 2

Chemistry, 24.06.2019 00:00
Kcal/moleee, e, eqsodium1s 2s 2p 3s111810911653he10034501002526magnesiumaluminium1s 2s 2p 3s1s 2s 2p 3s 3pwe27670411ce20 = 1what is the electron configuration when the valence electrons are removed from each of the elements listed in thchart? 1s22s22p3s21s 2s 2p60 15²2521s22s 2p 3s
Answers: 3
You know the right answer?
Excess ca(oh)₂ is shaken with water to produce a saturated solution. the solution is filtered, and a...
Questions







Geography, 12.08.2020 05:01




History, 12.08.2020 05:01






Mathematics, 12.08.2020 05:01



Spanish, 12.08.2020 05:01

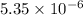 .
.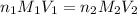
 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 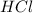
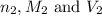 are the n-factor, molarity and volume of base which is
are the n-factor, molarity and volume of base which is 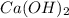 .
.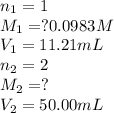
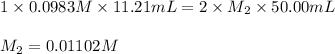
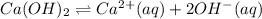
![[Ca^{2+}]=1\times 0.01102 M=0.01102 M](/tpl/images/0430/6322/b6c31.png)
![[OH^{-}]=2\times 0.01102 M=0.02204 M](/tpl/images/0430/6322/f1d7c.png)
![K_{sp}=[Ca^{2+}][OH^-]^2](/tpl/images/0430/6322/8de55.png)