Chemistry, 23.12.2019 17:31 akatherine1738
Mothballs are composed primarily of the hydrocarbon naphthalene (c10h8). when 1.025 g of naphthalene is burned in a bomb calorimeter, the temperature rises from 24.25 ∘c to 32.33 ∘c. find δerxn for the combustion of naphthalene. the heat capacity of the calorimeter, determined in a separate experiment, is 5.11kj/∘c.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2
You know the right answer?
Mothballs are composed primarily of the hydrocarbon naphthalene (c10h8). when 1.025 g of naphthalene...
Questions


Mathematics, 18.03.2021 03:20

English, 18.03.2021 03:20




Physics, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20

English, 18.03.2021 03:20



Mathematics, 18.03.2021 03:20


Mathematics, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20

Health, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20

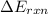 for combustion of naphthalene is -5164 kJ/mol
for combustion of naphthalene is -5164 kJ/mol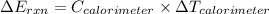
 refers change in temperature.
refers change in temperature.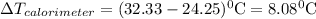
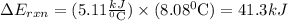
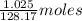 of naphthalene
of naphthalene