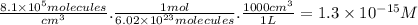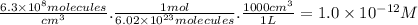The degradation of cf3ch2f (an hfc) by oh radicals in the troposphere is first order in each reactant and has a rate constant of k = 1.6 x 10^8 m^-1s^-1 at 4°c.
part a) if the tropospheric concentrations of oh and cf3ch2f are 8.1 x 10^5 and 6.3 x 10^8 molecules/cm^3, respectively, what is the rate of reaction at this temperature in m/s?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 22.06.2019 22:20
How do cfcs cause ozone depletion? how do cfcs cause ozone depletion? ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule.
Answers: 2

Chemistry, 23.06.2019 02:30
Asubstance is held in an open container. its particles move past one another at random speeds but do not leave the container. heat is removed from the system, and the particles slow down. when enough heat is removed, the particles no longer have enough speed to overcome the weak attractive forces between them. when this happens, the substance enters its solid state. the process described above is known as .
Answers: 3
You know the right answer?
The degradation of cf3ch2f (an hfc) by oh radicals in the troposphere is first order in each reactan...
Questions


Computers and Technology, 09.08.2019 23:20






Spanish, 09.08.2019 23:20

Mathematics, 09.08.2019 23:20

Biology, 09.08.2019 23:20


Biology, 09.08.2019 23:20

Advanced Placement (AP), 09.08.2019 23:20



Mathematics, 09.08.2019 23:20


Mathematics, 09.08.2019 23:20

History, 09.08.2019 23:20