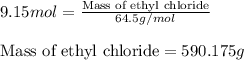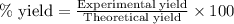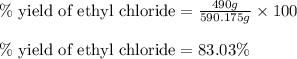Chemistry, 09.08.2019 23:20 4804397217
The reaction of ethane gas (c2h6) with chlorine gas (cl2) produces c2h5cl as its main product. calculate the percent yield of c2h5cl if the reaction of 300 g of ethane with 650 g of chlorine produced 490 g of c2h5cl .

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

You know the right answer?
The reaction of ethane gas (c2h6) with chlorine gas (cl2) produces c2h5cl as its main product. calcu...
Questions



Computers and Technology, 19.10.2019 02:30


Computers and Technology, 19.10.2019 02:30






Social Studies, 19.10.2019 02:30

Social Studies, 19.10.2019 02:30


History, 19.10.2019 02:30


Chemistry, 19.10.2019 02:30

English, 19.10.2019 02:30



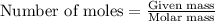 ....(1)
....(1)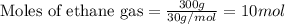
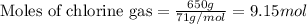
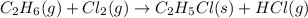
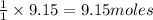 of ethane gas.
of ethane gas.