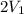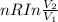Chemistry, 20.12.2019 03:31 arifkarimi9214
Calculate the change in the entropy of the system and also the change in the entropy of the surroundings, and the resulting total change in entropy, when a sample of nitrogen gas of mass 14 g at 298 k and 1.00 bar doubles its volume in (a) an isothermal reversible expansion, (b) an isothermal irreversible expansion against pext = 0, and (c) an adiabatic reversible expansion.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3


Chemistry, 23.06.2019 02:30
Ascientist wants to know how individual lions within a pride interact with each other in their own environment. to do this, the scientist sedates and tags all of the lions within a pride. then, he places several remotely-controlled video cameras near the lions' den and performs an observational field study. he collects continuous video footage over the span of one year, analyzes the video, and then forms conclusions based on his observations.
Answers: 2
You know the right answer?
Calculate the change in the entropy of the system and also the change in the entropy of the surround...
Questions



Mathematics, 28.12.2019 21:31


Geography, 28.12.2019 21:31





Health, 28.12.2019 21:31


Mathematics, 28.12.2019 21:31


Mathematics, 28.12.2019 21:31


Mathematics, 28.12.2019 21:31


Health, 28.12.2019 21:31

Mathematics, 28.12.2019 21:31

Mathematics, 28.12.2019 21:31

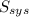 = 2.881 J/K; Δ
= 2.881 J/K; Δ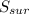 = -2.881 J/K; total change in entropy = 0
= -2.881 J/K; total change in entropy = 0 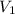
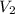 =
=