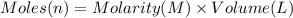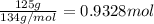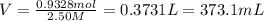You work for a cutlery manufacturer who wants to electrolytically precipitate 0.500 g of silver onto each piece of a batch of 250 forks. the preferred electrolytic solution for silver is agcn(aq). aqueous agcn is purchased as a 2.50 m solution. how many ml of agcn(aq) must be poured into your electrolysis vat to ensure you have sufficient ag to plate all of the forks

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

You know the right answer?
You work for a cutlery manufacturer who wants to electrolytically precipitate 0.500 g of silver onto...
Questions

Mathematics, 11.09.2019 04:30

Mathematics, 11.09.2019 04:30

Mathematics, 11.09.2019 04:30

History, 11.09.2019 04:30

Mathematics, 11.09.2019 04:30

World Languages, 11.09.2019 04:30


Mathematics, 11.09.2019 04:30

Mathematics, 11.09.2019 04:30


History, 11.09.2019 04:30