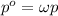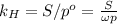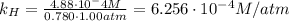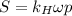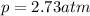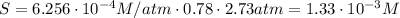Chemistry, 18.12.2019 04:31 terrysizemore666
Air is a mixture of gases that is about78.0percent n2 by volume. when air is at standard pressure and 25.0 degreec, the n2 component will dissolve in water with a solubility of 4.88 x 10^-4m. what is the value of henry's law constant for n2 under these conditions? express the constant numerically inmoles per liter per atmosphere. =6.26? 10^-4 mol/(l. atm) correct part b as a scuba diver descends under water, thepressure increases. at a total air pressure of 2.73 atm and a temperature of 25.0 degreec, what is the solubility of n2 in a diver's blood? [use the value of the henry's lawconstant calculated in part a, 6.26 x 10^-4mol/(l. atm). assume that the composition of the air in the tank is the sameas on land and that all of the dissolved nitrogen remains in theblood. express your answer numerically inmoles per liter. solubility =mol/l

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 22:30
Llama have 74 chromosomes how many chromosomes will they be found in their gametes explain how you know
Answers: 2

Chemistry, 22.06.2019 01:30
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3
You know the right answer?
Air is a mixture of gases that is about78.0percent n2 by volume. when air is at standard pressure an...
Questions






Computers and Technology, 19.08.2019 19:20

Computers and Technology, 19.08.2019 19:20

English, 19.08.2019 19:20





Mathematics, 19.08.2019 19:20

History, 19.08.2019 19:20


English, 19.08.2019 19:20


English, 19.08.2019 19:20

Mathematics, 19.08.2019 19:20

English, 19.08.2019 19:20

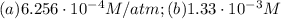
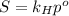
![p = 1.00 atm[/atm]Nitrogen's pecentage is:[tex]\omega = 0.780](/tpl/images/0423/6471/ab47c.png)