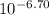Chemistry, 17.12.2019 04:31 Trishamw04
Astudent was given a 50.0 ml of 0.10 m solution of an unknown diprotic acid, h2a, which was titrated with 0.10 m naoh. after a total of 25.0 ml of naoh was added, the resulting solution has a ph of 6.70.
after a total of 50.0 ml of naoh was added, the ph of the solution was 8.00.
determine the values of ka1 and ka2 for the diprotic acid.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
How much energy moves onto the next level, in an energy pyramid
Answers: 1

Chemistry, 22.06.2019 00:30
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1
You know the right answer?
Astudent was given a 50.0 ml of 0.10 m solution of an unknown diprotic acid, h2a, which was titrated...
Questions


Mathematics, 17.10.2020 21:01

History, 17.10.2020 21:01



Mathematics, 17.10.2020 21:01


Mathematics, 17.10.2020 21:01

Chemistry, 17.10.2020 21:01

Mathematics, 17.10.2020 21:01

Mathematics, 17.10.2020 21:01


Mathematics, 17.10.2020 21:01




Mathematics, 17.10.2020 21:01


Mathematics, 17.10.2020 21:01

Computers and Technology, 17.10.2020 21:01