
Chemistry, 14.12.2019 07:31 live4dramaoy0yf9
Predicting nuclear stability is important when determining whether or not a nuclear reaction can take place spontaneously. use the following set of guidelines to predict the nuclear stability of the elements listed below.
1. stable nuclei have a high neutron-to-proton ratio, greater than 1.25 beyond atomic number 40 and continuing to rise at higher atomic numbers.
2. the majority of stable nuclei have even numbers of neutrons and protons. odd-even and even-odd combinations can be stable but stability is less likely.
3. nuclei that have the magic numbers of 2, 8, 20, 28, 50, or 82 protons or 2, 8, 20, 28, 50, 82 or 126 neutrons are more stable than nuclei that do not contain these numbers.
4. nuclei that have atomic numbers greater than 83 are unstable. nuclei are considered stable if one or more criteria are met.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 23.06.2019 04:00
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1

Chemistry, 23.06.2019 05:00
Activity two: just lemons, inc. production here's a one-batch sample of just lemons lemonade production. determine the percent yield and amount of leftover ingredients for lemonade production and place your answers in the data chart. hint: complete stoichiometry calculations for each ingredient to determine the theoretical yield. complete a limiting reactant-to-excess reactant calculation for both excess ingredients. water sugar lemon juice lemonade percent yield leftover ingredients 946.36 g 196.86 g 193.37 g 2050.25 g 89% just lemons lemonade recipe equation: 2 water + sugar + lemon juice = 4 lemonade mole conversion factors: 1 mole of water = 1 cup = 236.59 g 1 mole of sugar = 1 cup = 225 g 1 mole of lemon juice = 1 cup = 257.83 g 1 mole of lemonade = 1 cup = 719.42 g show your calculations below. analysis questions 1. based on taste observations only, which ingredients were in excess in the lemonade samples in activity one? in activity one the excess substances for each sample were the water and sugar. 2. based on the data in activity two, which excess ingredients are affecting the taste of the lemonade in the sample batch? 3. what can just lemons, inc. do during production to reduce the amount of excess ingredients and improve the taste of their lemonade? 4. try to reduce the amount of leftover ingredients by changing the amount of one, two, or all three starting ingredients. show your stoichiometric calculations below. 5. during factory inspection, just lemons, inc. discovered that a water valve to the lemonade mixing station was not functioning. once they repair it, more water will enter the mixing station. from what you know about the limiting and excess ingredients for current lemonade production, what advice would you give engineers about the upcoming increase in water?
Answers: 3
You know the right answer?
Predicting nuclear stability is important when determining whether or not a nuclear reaction can tak...
Questions




Biology, 01.07.2019 23:10


English, 01.07.2019 23:10




Mathematics, 01.07.2019 23:10




Mathematics, 01.07.2019 23:10

Spanish, 01.07.2019 23:10





Mathematics, 01.07.2019 23:10

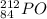
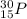
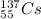
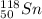
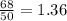 .It is more than 1.25.
.It is more than 1.25.

