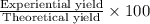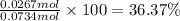Ammonia, nh3nh3 , can react with oxygen to form nitrogen gas and water. 4nh3(aq)+3o2(g)⟶2n2(g)+6h2o(l) 4nh3(aq)+3o2(g)⟶2n2(g)+6h2o(l) if 2.35 g2.35 g nh3nh3 reacts with 3.53 g3.53 g o2o2 and produces 0.650 l0.650 l n2n2 , at 295 k295 k and 1.01 bar1.01 bar , which reactant is limiting? o2(g)o2(g) nh3(aq)nh3(aq) calculate the percent yield of the reaction. percent yield:

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2
You know the right answer?
Ammonia, nh3nh3 , can react with oxygen to form nitrogen gas and water. 4nh3(aq)+3o2(g)⟶2n2(g)+6h2o(...
Questions




History, 19.11.2020 06:10



Mathematics, 19.11.2020 06:10

Mathematics, 19.11.2020 06:10

Biology, 19.11.2020 06:10

Mathematics, 19.11.2020 06:10

Business, 19.11.2020 06:10

Biology, 19.11.2020 06:10

Mathematics, 19.11.2020 06:10



Mathematics, 19.11.2020 06:10


Mathematics, 19.11.2020 06:10

Mathematics, 19.11.2020 06:10

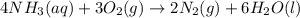
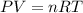
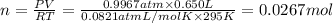
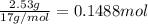
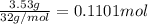
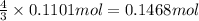 of ammonia.
of ammonia.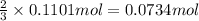 of nitrogen.
of nitrogen.