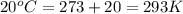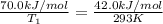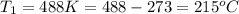Chemistry, 14.12.2019 01:31 ayoismeisjjjjuan
Apopular chemical demonstration is the "magic genie" procedure, in which hydrogen peroxide decomposes to water and oxygen gas with the aid of a catalyst. the activation energy of this (uncatalyzed) reaction is 70.0 kj/mol. when the catalyst is added, the activation energy (at 20.ºc) is 42.0 kj/mol. theoretically, to what temperature (ºc) would one have to heat the hydrogen peroxide solution so that the rate of the uncatalyzed reaction is equal to the rate of the catalyzed reaction at 20.ºc? assume the frequency factor a is constant, and assume the initial concentrations are the same. temperature = __ºc

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
You know the right answer?
Apopular chemical demonstration is the "magic genie" procedure, in which hydrogen peroxide decompose...
Questions

Mathematics, 17.10.2021 21:50

History, 17.10.2021 21:50

Mathematics, 17.10.2021 21:50





Mathematics, 17.10.2021 21:50



Mathematics, 17.10.2021 22:00

Mathematics, 17.10.2021 22:00


History, 17.10.2021 22:00

English, 17.10.2021 22:00

Mathematics, 17.10.2021 22:00


Social Studies, 17.10.2021 22:00

Chemistry, 17.10.2021 22:00

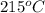
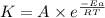
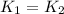
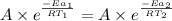
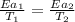 ..........(1)
..........(1)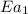 = activation energy for non-catalyzed reaction = 70.0 kJ/mol
= activation energy for non-catalyzed reaction = 70.0 kJ/mol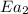 = activation energy for catalyzed reaction = 42.0 kJ/mol
= activation energy for catalyzed reaction = 42.0 kJ/mol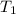 = temperature for non-catalyzed reaction = ?
= temperature for non-catalyzed reaction = ?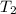 = temperature for catalyzed reaction =
= temperature for catalyzed reaction =