
Chemistry, 14.12.2019 01:31 orlando19882000
Calculate the ph of a 0.208 0.208 m solution of ethylenediamine ( h 2 nch 2 ch 2 nh 2 h2nch2ch2nh2). the p k a pka values for the acidic form of ethylenediamine ( h + 3 nch 2 ch 2 nh + 3 h3+nch2ch2nh3+) are 6.848 6.848 ( p k a1 pka1) and 9.928 9.928 ( p k a2 pka2). ph = ph= calculate the concentration of each form of ethylenediamine in this solution at equilibrium.
[ h 2 nch 2 ch 2 nh 2 ] =
[h2nch2ch2nh2]= m m
[ h 2 nch 2 ch 2 nh + 3 ] =
[h2nch2ch2nh3+]= m m
[ h + 3 nch 2 ch 2 nh + 3 ] =
[h3+nch2ch2nh3+]=

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5 m hcl? show all of the work needed to solve this problem. mg (s) + 2hcl (aq) → mgcl2 (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1

You know the right answer?
Calculate the ph of a 0.208 0.208 m solution of ethylenediamine ( h 2 nch 2 ch 2 nh 2 h2nch2ch2nh2)....
Questions






Biology, 08.04.2021 20:00

Mathematics, 08.04.2021 20:00

Biology, 08.04.2021 20:00



Mathematics, 08.04.2021 20:00

Mathematics, 08.04.2021 20:00



English, 08.04.2021 20:00


Chemistry, 08.04.2021 20:00

Mathematics, 08.04.2021 20:00

Mathematics, 08.04.2021 20:00

Mathematics, 08.04.2021 20:00

![[H_{2}NCH_{2}NCH_{2}NH_{2}]](/tpl/images/0417/9676/3fc2a.png) = 0.204 M;
= 0.204 M;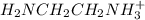 =
= 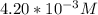
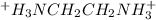 =
= 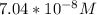
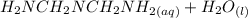 ⇔
⇔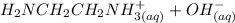
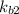 equation (1)
equation (1)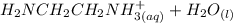 ⇔
⇔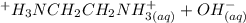
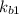 equation (2)
equation (2)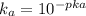
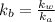

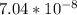
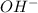 = x
= x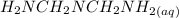 = 0.208-x
= 0.208-x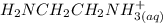 = x
= x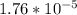
![[H_{2}NCH_{2}NCH_{2}NH_{2(aq)}]](/tpl/images/0417/9676/0b733.png) = 0.208 -
= 0.208 - 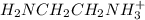
 = 2.38
= 2.38

