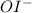Chemistry, 13.12.2019 09:31 20LindleyN
Which is an intermediate in this reaction mechanism: step 1: h2o2 + i- → h2o + oi- step 2: h2o2 + oi- → h2o + o2 + i- a. h2o2 b. i- c. oi- d. o2

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which term best describes the form sound takes as it travels away from a drum (a- gas)(b-music) ( c-waves) (d-particles
Answers: 3

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

You know the right answer?
Which is an intermediate in this reaction mechanism: step 1: h2o2 + i- → h2o + oi- step 2: h2o2 +...
Questions







Chemistry, 12.08.2020 08:01


Mathematics, 12.08.2020 08:01

Mathematics, 12.08.2020 08:01

Computers and Technology, 12.08.2020 08:01


Law, 12.08.2020 08:01


Mathematics, 12.08.2020 08:01


History, 12.08.2020 08:01


English, 12.08.2020 08:01

History, 12.08.2020 08:01