
Chemistry, 12.12.2019 05:31 david1236544
Three identical flasks contain three different gases at standard temperature and pressure. flask a contains flask b contains o3, and flask c contains n2. which flask contains the largest number of molecules? a) flask a b) flask b c) flask c d) all contain same number of molecules.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:10
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2


Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3
You know the right answer?
Three identical flasks contain three different gases at standard temperature and pressure. flask a c...
Questions


Mathematics, 20.10.2019 04:30




Chemistry, 20.10.2019 04:30


Social Studies, 20.10.2019 04:30

Chemistry, 20.10.2019 04:30


Biology, 20.10.2019 04:30

Biology, 20.10.2019 04:30

Social Studies, 20.10.2019 04:30



History, 20.10.2019 04:30


English, 20.10.2019 04:30


Mathematics, 20.10.2019 04:30

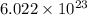 number of molecules
number of molecules

