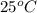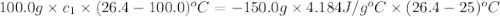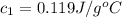Chemistry, 11.12.2019 18:31 lilquongohard
A100.0g sample of lead is heated to 100.0 oc (celsius) and is placed in a coffee cup calorimeter containing 150. g of water at 25.0 oc. after the metal cools, the final temperature of the metal and the water is 26.4 oc. calculate the specific heat capacity of lead from these experimental data, assuming that no heat escapes to the surroundings or is transferred to the calorimeter. specific heat of water = 4.184 j/g oc atomic mass pb = 207.2 g/mol calculate your answer in j/g oc with 3 significant figures, but enter the answer without units.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1
You know the right answer?
A100.0g sample of lead is heated to 100.0 oc (celsius) and is placed in a coffee cup calorimeter con...
Questions

Chemistry, 24.12.2019 03:31



















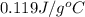
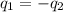
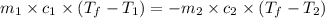
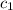 = specific heat of lead = ?
= specific heat of lead = ?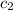 = specific heat of water =
= specific heat of water = 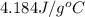
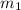 = mass of lead = 100.0 g
= mass of lead = 100.0 g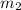 = mass of water = 150.0 g
= mass of water = 150.0 g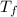 = final temperature =
= final temperature = 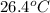
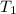 = initial temperature of lead =
= initial temperature of lead = 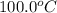
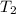 = initial temperature of water =
= initial temperature of water =