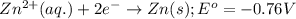Consider the following standard reduction potentials: zn2+(aq) + 2 e− latex: \longrightarrow⟶ zn(s) eo = −0.76 v mg2+(aq) + 2 e− latex: \longrightarrow⟶ mg(s) eo = −2.37 v ag+(aq) + e− latex: \longrightarrow⟶ ag(s) eo = +0.80 v which is the strongest oxidizing agent? group of answer choices

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 18:30
Amass of 100.0 g of solute is dissolved in water so that 850. ml of a 0.7500 m solution has been prepared. what is the molar mass of the solute?
Answers: 2

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
Consider the following standard reduction potentials: zn2+(aq) + 2 e− latex: \longrightarrow⟶ zn(s...
Questions





Mathematics, 06.07.2020 09:01

Mathematics, 06.07.2020 09:01

Mathematics, 06.07.2020 09:01

Mathematics, 06.07.2020 09:01









Computers and Technology, 06.07.2020 09:01

Mathematics, 06.07.2020 09:01


Mathematics, 06.07.2020 09:01

 potential will always get reduced and will undergo reduction reaction easily.
potential will always get reduced and will undergo reduction reaction easily.