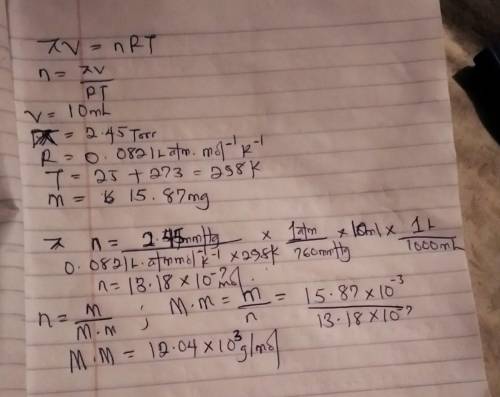Chemistry, 06.07.2020 09:01 junielouwho
The osmotic pressure of a solution containing 15.87 mg of an unknown protein per 10.0 mL of solution is 2.45 torr at 25oC. Find the molar mass of the unknown protein.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 23.06.2019 09:00
A2-kg bowling ball is 1 meter off the ground on a post when it falls. just before it reaches the ground,its traveling 4.4 m/s. assuming that there is no air resistant, which statement is true a. the initial potential energy is less then the final kinetic energy b. the mechanical energy is not conserved c. the mechanical energy is conserved d. the initial potential energy is greater than the final kinetic energy
Answers: 3

Chemistry, 23.06.2019 12:30
D5w is shorthand for a 5% glucose rehydration fluid used in ivs. the doctor orders 1200 ml d5w@ 30 gtts/min. you have an iv tube that delivers 18 gtts/cc. how many hours will it take for the 1200 cc bottle to infuse?
Answers: 2
You know the right answer?
The osmotic pressure of a solution containing 15.87 mg of an unknown protein per 10.0 mL of solution...
Questions

Mathematics, 29.10.2020 05:00



Biology, 29.10.2020 05:00


Chemistry, 29.10.2020 05:00

Mathematics, 29.10.2020 05:00

Mathematics, 29.10.2020 05:00

Mathematics, 29.10.2020 05:00

Mathematics, 29.10.2020 05:00

History, 29.10.2020 05:00


Computers and Technology, 29.10.2020 05:00

Mathematics, 29.10.2020 05:00




History, 29.10.2020 05:00