
Chemistry, 10.12.2019 05:31 kimberlyhansen92
Acertain liquid x has a normal boiling point of 108.30 °c and a boiling point elevation constant kb=1.07 °c kg/mol. a solution is prepared by dissolving some ammonium sulfate (nh4)2 so4 in 600. g of x. this solution boils at 109.7 °c. calculate the mass of (nh4)2 so4 that was dissolved. be sure your answer is rounded to the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2


Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2
You know the right answer?
Acertain liquid x has a normal boiling point of 108.30 °c and a boiling point elevation constant kb=...
Questions



Mathematics, 11.07.2019 06:00


English, 11.07.2019 06:00

Social Studies, 11.07.2019 06:00


Mathematics, 11.07.2019 06:00


Social Studies, 11.07.2019 06:00


History, 11.07.2019 06:00


Social Studies, 11.07.2019 06:00


Mathematics, 11.07.2019 06:00



Spanish, 11.07.2019 06:00

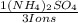 = 0,262 moles of (NH₄)₂SO₄
= 0,262 moles of (NH₄)₂SO₄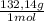 = 34,6g of (NH₄)₂SO₄
= 34,6g of (NH₄)₂SO₄

