
Chemistry, 10.12.2019 05:31 annabanana1298
1‑propanol ( n ‑propanol) and 2‑propanol (isopropanol) form ideal solutions in all proportions. calculate the partial pressure and the mole fraction ( y ) of the vapor phase of each component in equilibrium with each of the given solutions at 25 °c. p ∘ prop = 20.9 torr and p ∘ iso = 45.2 torr at 25 °c. a solution with a mole fraction of x prop = 0.247 .

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 23.06.2019 07:30
Assignment directions: pick one of the following chemists and perform a bit of research on him/her. answer the following questions. alice hamilton rosalind franklin marie curie gertrude b. elion ada yonath henry cavendish robert boyle antoine lavoisier mario j. molina svante arrhenius
Answers: 1

Chemistry, 23.06.2019 08:00
Problem page a jet airplane reaches 846. km/h on a certain flight. what distance does it cover in 13.0 min? set the math up. but don't do any of it. just leave your answer as a math expression. also, be sure your answer includes all the correct unit symbols.
Answers: 2

Chemistry, 23.06.2019 11:30
Jenny places a strip of ph paper into a solution. when she removes the ph paper, it has turned yellow-green. what should jenny do next to determine the ph of her solution? a. use a different testing method because the ph paper should not change colors b. place the ph paper into a machine that reads the ph of the solution c. compare the ph paper's color with the color of ph paper from another solution d. compare the ph paper's color with a chart of colors and ph ranges
Answers: 1
You know the right answer?
1‑propanol ( n ‑propanol) and 2‑propanol (isopropanol) form ideal solutions in all proportions. calc...
Questions





Computers and Technology, 17.07.2019 14:00



History, 17.07.2019 14:00



Chemistry, 17.07.2019 14:00






History, 17.07.2019 14:00



History, 17.07.2019 14:00

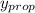 = 0.134;
= 0.134; 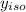 = 0.866
= 0.866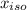 ) = 1 - mole fraction of propanol (
) = 1 - mole fraction of propanol (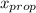 ).
). 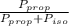
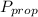 is the partial pressure of propanol and
is the partial pressure of propanol and 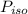 is the partial pressure of isopropanol.
is the partial pressure of isopropanol.

