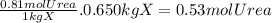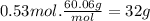Chemistry, 10.12.2019 01:31 Leanylopez0811
Acertain substance x has a normal boiling point of 124.2 °c and a molal boiling point elevation constant k,-06-0c-kg-mol ·a solution is prepared by dissolving some urea ((n112)co) in 650. g ofl. this solution boils at 124.7 oc, calculate the mass of urea that was dissolved. be sure your answer has the correct number of significant digits. 31.48 g

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1
You know the right answer?
Acertain substance x has a normal boiling point of 124.2 °c and a molal boiling point elevation cons...
Questions

Mathematics, 06.11.2019 23:31



English, 06.11.2019 23:31




Social Studies, 06.11.2019 23:31

Mathematics, 06.11.2019 23:31



Chemistry, 06.11.2019 23:31


Chemistry, 06.11.2019 23:31


Business, 06.11.2019 23:31




Mathematics, 06.11.2019 23:31