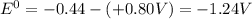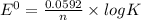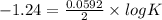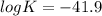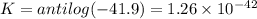Chemistry, 10.12.2019 01:31 sassycutie523
Eo cell = 0.0592 v n log(k) calculate the equilibrium constant for the following reaction at 25°c: 2ag(s) + fe2+(aq) ⇌ 2ag+(aq) + fe(s) enter your answer in scientific notation. k = × 10

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:40
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3

You know the right answer?
Eo cell = 0.0592 v n log(k) calculate the equilibrium constant for the following reaction at 25°c:...
Questions

Mathematics, 02.10.2020 15:01

Mathematics, 02.10.2020 15:01

Social Studies, 02.10.2020 15:01

Mathematics, 02.10.2020 15:01




English, 02.10.2020 15:01

Physics, 02.10.2020 15:01

Geography, 02.10.2020 15:01




English, 02.10.2020 15:01

Health, 02.10.2020 15:01

Business, 02.10.2020 15:01

Business, 02.10.2020 15:01

History, 02.10.2020 15:01


Mathematics, 02.10.2020 15:01

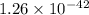
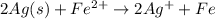
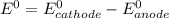
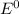 are standard reduction potentials.
are standard reduction potentials.
![E^0_{[Fe^{2+}/Fe]}=-0.44V](/tpl/images/0410/9384/59478.png)
![E^0_{[Ag^{+}/Ag]}=+0.80V](/tpl/images/0410/9384/76e17.png)
![E^0=E^0_{[Fe^{2+}/Fe]}- E^0_{[Ag^{+}/Ag]}](/tpl/images/0410/9384/81b1e.png)