

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Which object forms when a supergiant runs out of fuel? a red giant a black hole a white dwarf a neutron star
Answers: 1

Chemistry, 21.06.2019 22:30
The diagram shows the structures of horse and cat forelimbs. what does the diagram suggest about the evolutionary relationship between these two mammals? a. they have homologous structures, indicating a common ancestor. b. they have analogous structures, indicating a common ancestor. c. they have homologous structures, indicating that they do not have a common ancestor. d. they have analogous structures, indicating that they do not have a common ancestor.
Answers: 2

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1
You know the right answer?
Given that kp = 3.5 x 10-4 for the reaction 2 co(g) < => c(graphite) + co2(g), what is the pa...
Questions



Mathematics, 15.12.2020 23:20





Biology, 15.12.2020 23:20


Mathematics, 15.12.2020 23:20


Biology, 15.12.2020 23:20

Chemistry, 15.12.2020 23:20

Chemistry, 15.12.2020 23:20

Chemistry, 15.12.2020 23:20

Spanish, 15.12.2020 23:20


English, 15.12.2020 23:20


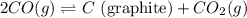
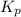 for above reaction follows:
for above reaction follows: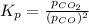
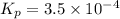
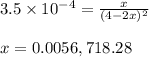
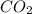 = 0.0056 atm
= 0.0056 atm

