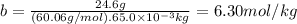Acertain substance condenses at a temperature of 123.3°c . but if a 65.0 gm sample of x is prepared with 24.6g of urea ((nh2)2co) dissolved in it, the sample is found to have a condensation point of 124.3°c instead.
1. calculate the molal boiling point elevation constant kb of x . round your answer to significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3

Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1
You know the right answer?
Acertain substance condenses at a temperature of 123.3°c . but if a 65.0 gm sample of x is prepared...
Questions

Mathematics, 03.08.2020 14:01


Chemistry, 03.08.2020 14:01


Mathematics, 03.08.2020 14:01


Mathematics, 03.08.2020 14:01

Biology, 03.08.2020 14:01



Physics, 03.08.2020 14:01

Advanced Placement (AP), 03.08.2020 14:01

Geography, 03.08.2020 14:01

Mathematics, 03.08.2020 14:01


Mathematics, 03.08.2020 14:01


Business, 03.08.2020 14:01

Mathematics, 03.08.2020 14:01

Mathematics, 03.08.2020 14:01