
Chemistry, 09.12.2019 20:31 andrewmena05
What is the rate law for the following mechanism? step1: step2: a+bb+c⇌→cd(fast)(slow) express your answer in terms of k and the necessary concentrations (e. g., k[a]^3[d]).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3

Chemistry, 23.06.2019 02:00
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2

Chemistry, 23.06.2019 02:20
Why dose heating increase the speed at which a solution dissolved in water
Answers: 1
You know the right answer?
What is the rate law for the following mechanism? step1: step2: a+bb+c⇌→cd(fast)(slow) express your...
Questions




















![Rate=k'[A][B]^2](/tpl/images/0410/3837/02776.png)
 (fast)
(fast)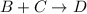 (slow)
(slow)![Rate=k[B][C]](/tpl/images/0410/3837/2d91f.png) ............(1)
............(1)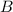 , we get:
, we get:![K=\frac{[C]}{[A][B]}](/tpl/images/0410/3837/f1e20.png)
![[C]=K\times [A][B]](/tpl/images/0410/3837/91a27.png) ...........(2)
...........(2)![Rate=k[B]\times K\times [A][B]](/tpl/images/0410/3837/d3849.png)
![Rate=K\times k[A][B]^2](/tpl/images/0410/3837/56d48.png)



