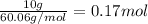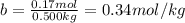Chemistry, 09.12.2019 20:31 alex12everett
The molal freezing point depression constant =kf6.19·°c·kgmol−1 for a certain substance x . when 10.g of urea nh22co are dissolved in 500.g of x , the solution freezes at −6.8°c . calculate the freezing point of pure x . be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1


Chemistry, 23.06.2019 16:30
Ashell can hold a maximum of 32 electrons, what is the value of n?
Answers: 3

You know the right answer?
The molal freezing point depression constant =kf6.19·°c·kgmol−1 for a certain substance x . when 10....
Questions

Social Studies, 07.11.2020 21:10

Business, 07.11.2020 21:10

Mathematics, 07.11.2020 21:10





Physics, 07.11.2020 21:10


Mathematics, 07.11.2020 21:20

History, 07.11.2020 21:20

Social Studies, 07.11.2020 21:20



Biology, 07.11.2020 21:20


Arts, 07.11.2020 21:20

Mathematics, 07.11.2020 21:20