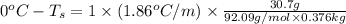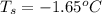Chemistry, 09.12.2019 19:31 nayelimoormann
Determine the freezing point depression of a solution that contains 30.7 g glycerin (c3h8o3, molar mass = 92.09 g/mol) in 376 ml of water. some possibly useful constants for water are kf = 1.86°c/m and kb = 0.512°c/m.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
If a reaction has g of -136kj at 110°c, will it be spontaneous at this temperature (110°c)? yes or no
Answers: 2

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 02:30
24 points and brainliest to anyone who can answer under 10 minutes with best ! the table below shows the role of different substances during photosynthesis. substance role during photosynthesis glucose stores chemical energy water combines with glucose to form carbon dioxide chlorophyll traps sunlight which of the following statements would correct one of the roles listed in the table? glucose combines with carbon to form water. chlorophyll reacts with light to produce carbon dioxide. water combines with carbon dioxide during photosynthesis. chlorophyll stores chemical energy needed for photosynthesis.
Answers: 1

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1
You know the right answer?
Determine the freezing point depression of a solution that contains 30.7 g glycerin (c3h8o3, molar m...
Questions




Mathematics, 17.12.2020 23:30


Mathematics, 17.12.2020 23:30

Chemistry, 17.12.2020 23:30

Computers and Technology, 17.12.2020 23:30


Mathematics, 17.12.2020 23:30


Mathematics, 17.12.2020 23:30



Biology, 17.12.2020 23:30

Chemistry, 17.12.2020 23:30


History, 17.12.2020 23:30


Biology, 17.12.2020 23:30

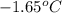
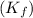 for water =
for water = 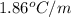

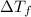 = change in freezing point
= change in freezing point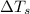 = freezing point of solution = ?
= freezing point of solution = ?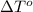 = freezing point of water =
= freezing point of water = 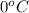
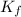 = freezing point constant for water =
= freezing point constant for water =