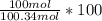Chemistry, 05.12.2019 05:31 goreimani9
Pure chlorobenzene is contained in a flask attached to an open-end mercury manometer. when the flask contents are at 58.3°c, the height of the mercury in the arm of the manometer connected to the flask is 747 mm, and that in the arm open to the atmosphere is 52 mm. at 110°c, the mercury level is 577 mm in the arm connected to the flask, and 222 mm in the other arm. atmospheric pressure is 755 mmhg. (a) using the data given, find δhv and b in the clausius-clapeyron equation. (b) air saturated with chlorobenzene at 130°c and 101.3kpa (absolute) is cooled to 58.3°c at constant pressure. estimate the percentage of the chlorobenzene originally in the vapor that condenses. (hint: draw a flowchart with separate vapor and liquid streams)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1
You know the right answer?
Pure chlorobenzene is contained in a flask attached to an open-end mercury manometer. when the flask...
Questions

History, 10.09.2019 22:30


Mathematics, 10.09.2019 22:30









Computers and Technology, 10.09.2019 22:30








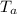 = 58.3°C
= 58.3°C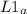 = 747mmHg
= 747mmHg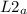 = 52mmHg
= 52mmHg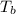 = 110°C
= 110°C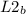 = 222mmHg
= 222mmHg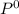 =
= 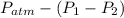
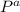 = 755mmHg - (747mmHg - 52mmHg)
= 755mmHg - (747mmHg - 52mmHg)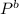 = 755mmHg - (577mmHg = 222mmHg)
= 755mmHg - (577mmHg = 222mmHg)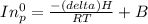
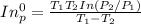
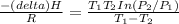
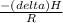 =
= 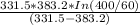
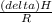 = 4661.4k
= 4661.4k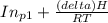
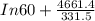
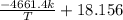
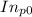 =
= 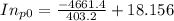
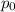 =
= 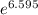
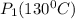 = 731.44mmHg
= 731.44mmHg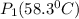 = 60mmHg
= 60mmHg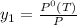
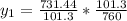
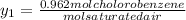
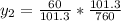
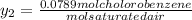
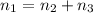
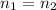 + 100mol
+ 100mol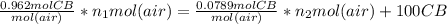
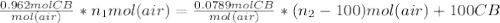
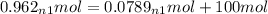
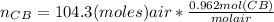
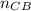 = 100.34mol CB
= 100.34mol CB