
Gaseous methane (ch4) reacts with gaseous oxygen gas (02) to produce gaseous carbon dioxide (co2) and gaseous water (h20). what is the theoretical yield of carbon dioxide formed from the reaction of 1.28 g of methane and 10.1 g of oxygen gas? be sure your answer has the correct number of significant digits in it. 02

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Mitosis is a type of cell division that produces cells that are identical to the parent cell. meiosis is a different type of cell division that produces cells that carry have a genetic material of the parent cell. based on the information provided how do the purpose of mitosis and meiosis differ
Answers: 3


Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

You know the right answer?
Gaseous methane (ch4) reacts with gaseous oxygen gas (02) to produce gaseous carbon dioxide (co2) an...
Questions

Physics, 05.05.2021 14:00





Physics, 05.05.2021 14:00

English, 05.05.2021 14:00




Mathematics, 05.05.2021 14:00


Chemistry, 05.05.2021 14:00

Mathematics, 05.05.2021 14:00

Mathematics, 05.05.2021 14:00


Mathematics, 05.05.2021 14:00



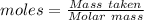
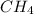 :-
:- 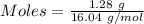
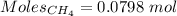
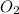 :-
:-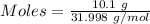
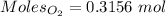
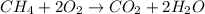
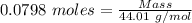
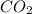 = 3.51 g
= 3.51 g

