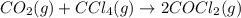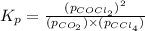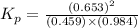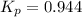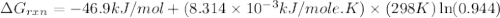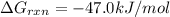Chemistry, 04.12.2019 00:31 jonquil201
Consider the reaction: co2(g) + ccl4(g) ⇌ 2 cocl2(g) δg° = 46.9 kj under the following conditions at 25 oc: latex: p_{co_2}p c o 2= 0.459 atm, latex: p_{ccl_4}p c c l 4= 0.984 atm, and latex: p_{cocl_2}p c o c l 2= 0.653 atm, δg for the reaction is , and the forward the reaction is

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1
You know the right answer?
Consider the reaction: co2(g) + ccl4(g) ⇌ 2 cocl2(g) δg° = 46.9 kj under the following conditions a...
Questions


Social Studies, 18.03.2021 01:20

Mathematics, 18.03.2021 01:20

Mathematics, 18.03.2021 01:20

English, 18.03.2021 01:20


Mathematics, 18.03.2021 01:20





Mathematics, 18.03.2021 01:20


Mathematics, 18.03.2021 01:20

English, 18.03.2021 01:20


Physics, 18.03.2021 01:20


English, 18.03.2021 01:20

Mathematics, 18.03.2021 01:20

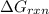 is,
is, 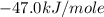
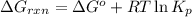 ............(1)
............(1)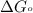 = standard Gibbs free energy = 46.9 kJ
= standard Gibbs free energy = 46.9 kJ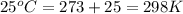
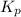 = equilibrium constant
= equilibrium constant