
Chemistry, 04.12.2019 00:31 anisagreen10
A0.158 g sample of magnesium metal reacts completely with 100.0 ml of 1.0 m hydrochloric acid in a coffee cup calorimeter. the temperature of the solution rose from 25.6°c to 32.8°c. what is ∆hrxn? assume the specific heat of the solution is 4.184 j/mol-k and the density is 1.0 g/ml.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1
You know the right answer?
A0.158 g sample of magnesium metal reacts completely with 100.0 ml of 1.0 m hydrochloric acid in a c...
Questions




Biology, 05.07.2019 15:50


English, 05.07.2019 15:50

Mathematics, 05.07.2019 15:50

History, 05.07.2019 15:50



Biology, 05.07.2019 16:00

Social Studies, 05.07.2019 16:00



Social Studies, 05.07.2019 16:00




Mathematics, 05.07.2019 16:00

Mathematics, 05.07.2019 16:00

 =
= 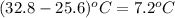
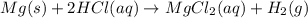
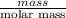
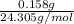
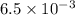
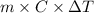
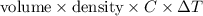
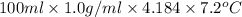
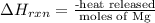
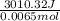
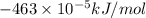 (as 1 kJ = 1000 J)
(as 1 kJ = 1000 J) kJ/mol.
kJ/mol. 

