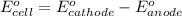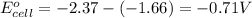Consider a galvanic cell based on the reaction al^3+_(aq) + mg_(s) rightarrow al_(s) + mg^2+ _(aq) the half-reactions are al^3+ + 3 e^- rightarrow al e degree = - 1.66 v mg^2+ + 2 e^- rightarrow mg e degree = - 2.37 v give the balanced cell reaction and calculate e degree for the cell.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:30
If a 60-g object has a volume of 30 cm3, what is its density? 2 g/cm3 0.5 cm3/g 1800 g * cm3 none of the above
Answers: 3

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1
You know the right answer?
Consider a galvanic cell based on the reaction al^3+_(aq) + mg_(s) rightarrow al_(s) + mg^2+ _(aq) t...
Questions





Mathematics, 12.01.2022 07:50

SAT, 12.01.2022 07:50


Social Studies, 12.01.2022 07:50

SAT, 12.01.2022 07:50

Health, 12.01.2022 07:50



Physics, 12.01.2022 07:50



Social Studies, 12.01.2022 07:50



Geography, 12.01.2022 07:50

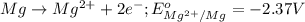 ( × 3)
( × 3)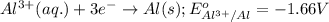 ( × 2)
( × 2)
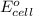 of the reaction, we use the equation:
of the reaction, we use the equation: