
Chemistry, 03.12.2019 22:31 allieballey0727
Given that h 2 ( g ) + f 2 ( g ) ⟶ 2 hf ( g ) δ h ∘ rxn = − 546.6 kj 2 h 2 ( g ) + o 2 ( g ) ⟶ 2 h 2 o ( l ) δ h ∘ rxn = − 571.6 kj calculate the value of δ h ∘ rxn for 2 f 2 ( g ) + 2 h 2 o ( l ) ⟶ 4 hf ( g ) + o 2 ( g )

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:40
What kind of ion is contained in salts that produce an acidic solution? a positive ion that attracts a proton from water a positive ion that releases a proton to water a negative ion that attracts a proton from water a negative ion that releases a proton to water
Answers: 1

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 23.06.2019 05:30
Scientist think that animals with remarkably similar embryo development probably shared a common ancestor
Answers: 1
You know the right answer?
Given that h 2 ( g ) + f 2 ( g ) ⟶ 2 hf ( g ) δ h ∘ rxn = − 546.6 kj 2 h 2 ( g ) + o 2 ( g ) ⟶ 2 h 2...
Questions








Mathematics, 29.08.2020 20:01




Chemistry, 29.08.2020 20:01


English, 29.08.2020 20:01





English, 29.08.2020 20:01

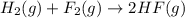
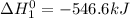 (1)
(1)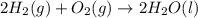
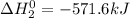 (2)
(2)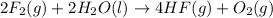
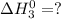 (3)
(3)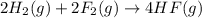
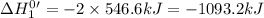 (1')
(1')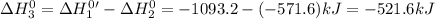 .
.

