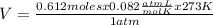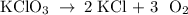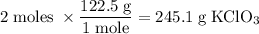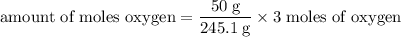Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:00
The density of a planet is 0.69 g/cm3 (density of water is 1.0 g/cm3). which of the following planets might this be? a. mercury b. venus c. saturn d. mars
Answers: 3

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3

Chemistry, 23.06.2019 01:30
Adirect relationship can be represented by: a curve a pie chart
Answers: 2
You know the right answer?
Determine the volume of o2 (at stp) formed when 50.0 g of kclo3 decomposes according to the followin...
Questions

Chemistry, 31.08.2019 00:20













Mathematics, 31.08.2019 00:30






Mathematics, 31.08.2019 00:30

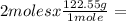 245.1 grams of KClO₃
245.1 grams of KClO₃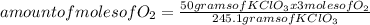
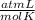 T= 0 C= 273 K
T= 0 C= 273 K