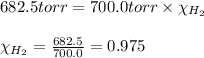Chemistry, 03.12.2019 18:31 taridunkley724
A30.0 ml sample of hydrogen gas (h2) is collected over water at 20.00∘c and has a total pressure of 700.0 torr. the partial pressure of water vapor at 20.00∘c is 17.5 torr. calculate the mole fraction of h2 gas in the sample.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:10
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2
You know the right answer?
A30.0 ml sample of hydrogen gas (h2) is collected over water at 20.00∘c and has a total pressure of...
Questions



Social Studies, 21.01.2020 01:31

















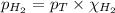
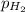 = partial pressure of hydrogen gas = 682.5 torr
= partial pressure of hydrogen gas = 682.5 torr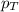 = total pressure = 700.0 torr
= total pressure = 700.0 torr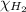 = mole fraction of hydrogen gas = ?
= mole fraction of hydrogen gas = ?