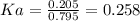Chemistry, 30.11.2019 05:31 lefthandeddolan
An acid-base indicator, hln, dissociates according to the following reaction in an aqueous solution. hinlag) in (aq) h (aq) the protonated form of the indicator, hln, has a molar absorptivity of 2929 m cm 1 and the deprotonated form, in has a molar absorptivity of 20060 m-1. cm 1 at 440 nm. the ph of a solution containing a mixture of hin and in s adjusted to 6.12. the total concentration of hin and in s 0.000127 m. the absorbance of this solution was measured at 440 nm in a 1.00 cm cuvette and was determined to be 0.818. calculate pka for hin.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 23.06.2019 03:30
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus.b) the number of neutrons it contains in its nucleus.c) the number of protons it has in a cloud around the nucleus.d) the number of neutrons it has in a cloud around the nucleus.e) the number of electrons it exchanges with its neighbors.
Answers: 1

Chemistry, 23.06.2019 07:30
Which of the following statements best explains why chemistry is testable a) it can measure data by experiments b) it cannot add new evidence c) it cannot be verified d) it is biased
Answers: 1

Chemistry, 23.06.2019 08:40
A20 liter cylinder of helium at a pressure of 150 atm and a temperature of 27°c is used to fill a balloon at 1.00 atm and 37°c. what is the volume of the balloon? a. 0.14 liters b. 3000 liters c. 2900 liters d. 2400 liters e. 3100 liters
Answers: 1
You know the right answer?
An acid-base indicator, hln, dissociates according to the following reaction in an aqueous solution....
Questions



Mathematics, 31.03.2021 21:40


Mathematics, 31.03.2021 21:40



Biology, 31.03.2021 21:40




Mathematics, 31.03.2021 21:40


Mathematics, 31.03.2021 21:40

Social Studies, 31.03.2021 21:40

Mathematics, 31.03.2021 21:40

Biology, 31.03.2021 21:40


Mathematics, 31.03.2021 21:40

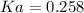
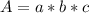
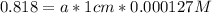

![x_{HIn]+x_{In}=1](/tpl/images/0397/0230/d5361.png)
![x_{HIn]=1-x_{In}](/tpl/images/0397/0230/0ee73.png)
![a=x_{In}*20060cm^{-1}*M^{-1}+ x_{HIn]*2929cm^{-1}*M^{-1}](/tpl/images/0397/0230/9addd.png)
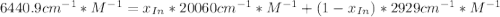
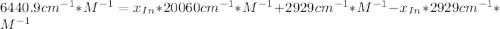
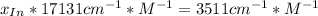
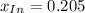
![x_{HIn]=1-0.205=0.795](/tpl/images/0397/0230/0ce5b.png)
![Ka=\frac{[In]}{[HIn]}](/tpl/images/0397/0230/065b2.png)