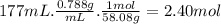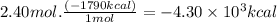Chemistry, 30.11.2019 05:31 gslovestodance6879
Consider the following thermochemical equation for the combustionof acetone, c3h6o, the main ingredient innail polish remover. c3h6o(l) + 4o2 (g) > 3co2 (g) + 3h2o (g), δhoof the reaction = -1790 kcalif a bottle of nail polish remover contains 177 ml of acetone, how much heat would be released by its complete combustion? thedensity of acetone is 0.788 g/ml.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

You know the right answer?
Consider the following thermochemical equation for the combustionof acetone, c3h6o, the main ingredi...
Questions

Mathematics, 29.07.2019 00:50


History, 29.07.2019 00:50

Geography, 29.07.2019 00:50




Geography, 29.07.2019 00:50

Physics, 29.07.2019 00:50

English, 29.07.2019 00:50



Mathematics, 29.07.2019 00:50



Mathematics, 29.07.2019 00:50



English, 29.07.2019 00:50

Mathematics, 29.07.2019 00:50