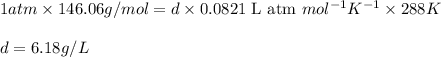Chemistry, 30.11.2019 05:31 lolsmaster3951
Calculate to three significant digits the density of sulfur hexafluoride gas at exactly 15°c and exactly 1atm . you can assume sulfur hexafluoride gas behaves as an ideal gas under these conditions.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1
You know the right answer?
Calculate to three significant digits the density of sulfur hexafluoride gas at exactly 15°c and exa...
Questions

Mathematics, 07.07.2019 14:00

Mathematics, 07.07.2019 14:00

World Languages, 07.07.2019 14:00



Arts, 07.07.2019 14:00

History, 07.07.2019 14:00


Mathematics, 07.07.2019 14:00

Mathematics, 07.07.2019 14:00

Spanish, 07.07.2019 14:00

English, 07.07.2019 14:00

Spanish, 07.07.2019 14:00



History, 07.07.2019 14:00

Mathematics, 07.07.2019 14:00


Mathematics, 07.07.2019 14:00

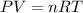
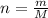
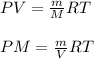
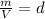 which is known as density of the gas
which is known as density of the gas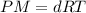 .....(1)
.....(1)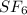 = 146.06 g/mol
= 146.06 g/mol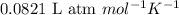
![15^oC=[15+273]K=288K](/tpl/images/0397/0399/b3c00.png)