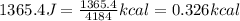Chemistry, 30.11.2019 02:31 kyleescott8857
1. convert 1365.4 joules into kilocalories.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
1. convert 1365.4 joules into kilocalories....
Questions

Social Studies, 13.10.2020 01:01





Mathematics, 13.10.2020 01:01

Mathematics, 13.10.2020 01:01




English, 13.10.2020 01:01


History, 13.10.2020 01:01

Social Studies, 13.10.2020 01:01




Geography, 13.10.2020 01:01

Biology, 13.10.2020 01:01

Mathematics, 13.10.2020 01:01